Unfair Trading
The purpose of this post is to reveal a few samples of things that are taught on a homeopathy ‘degree’ course. The course in question was the "BSc Hons homeopathy course at the University of Central Lancashire (UCLAN). Entry to this course was closed in 2008 and, after an internal review, UCLAN closed almost all of the rest of its courses in alternative medicine too. The university is to be commended for this .
The purpose of making public some of what used to be taught is not to embarrass UCLAN, which has already done the sensible thing, but to make it clear that the sort of thing taught on such courses is both absurd and dangerous, in the hope of discouraging other courses
|
.Three years after I first asked for teaching materials, the Information Commisioner ruled that all the reasons given for refusal were invalid, and they must be handed over. However UCLAN then appealed against the decision, so the appeal went to an Information Tribunal. That appeal was lost decisively and UCLAN was.obliged to provide the whole of the course material. On Christmas Eve I got five large box files, 13.7 kg of documents, or 30 pounds, in old money. |

|
Because these documents are copyright, I rely on the twin defences of fair quotation (only a tiny proportion is being quoted) and public interest. The Information Tribunal decided very firmly that it was in the public interest that it should be known what is taught on such courses, and that can be achieved if some of it is made public. Here are a few extracts.
Code of ethics
The students are given a copy of the code of ethics of the Society of Homeopaths. This is 25 pages long, but paragraph 48 is especially interesting.
48 Advertisements, stationery and name plates maintain a high standard of propriety and
integrity to enhance the reputation of homeopathy.
- Advertising shall not contain claims of superiority.
- No advertising may be used which expressly or implicitly claims to cure named diseases.
- Advertising shall not be false, fraudulent, misleading, deceptive, extravagant or sensational.
No mention though, of the fact that this code of ethics has been repeatedly breached by the Society of Homeopaths itself, on its own website. See, for example, here in 2007 and again in 2009. as well as Ernst’s article on this topic.
Anyone who has followed dialogues among homeopaths knows that "claims to cure named diseases" is the norm not the exception. The code of ethics is just a bad joke. And the (late) course at UCLAN was no exception. Take, for example, course HP3002, Therapeutic Homeopathy, module leader Jean Duckworth.
Homeopathic treatment of cancer
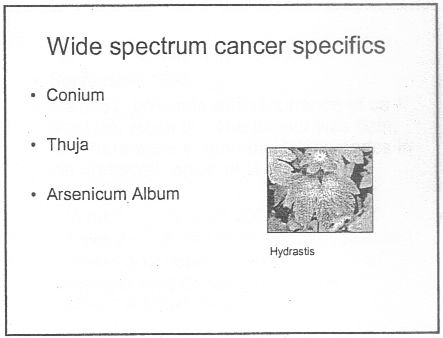
There was a lecture on HP3002 called "A Homeopathic Approach to Cancer (Ramakrishnan methodology [sic])".. Here are 10 slides from that lecture. It is illegal to claim to be able to cure cancer under the Cancer Act 1939. If a homeopath were to make claims like these in public they’d be open to prosecution, not to mention in breach of the SoH’s code of ethics. If cancer is not a "named disease", what is?

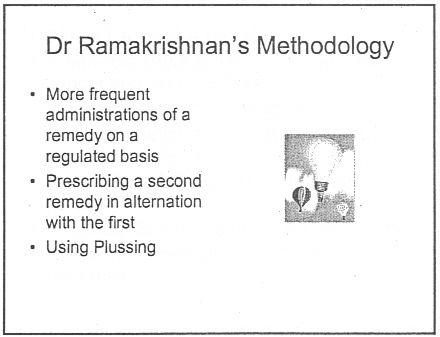

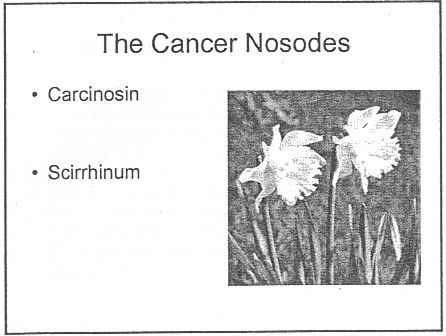
Specific treatments for a named disease are recommended.
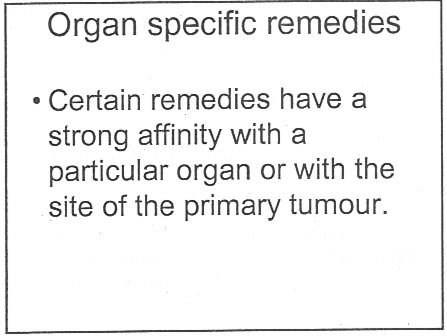
What happened to treating the whole person? Now specific organs are being treated. The term "affinity", as used here, is of course sheer hocus pocus.

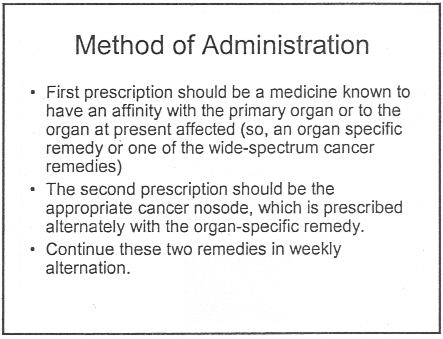
It is easy to forget when reading this that none of the “medicines” contain any medicine whatsoeever.
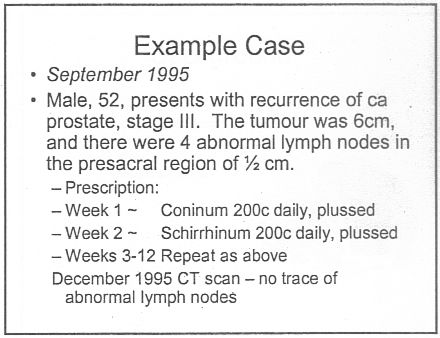
Notice that the term "remedy" is used throughout. Any reasonable person would interpret "remedy" to imply "cure", though no doubt a homeopath, if challenged, would claim that "remedy" carried no such implication. The last slide is typical of junk medicine: the personal testimonial, supplied with no detail whatsoever. Just an anecdote which is useless as evidence.
This lecture alone strikes me as a cruel (and possibly illegal) hoax perpetrated on desperate patients. Of course a true believer might get some solace from taking the sugar pills, but that is not sufficient justification.
The same course dealt with quite a lot of other "named diseases", autism, ADHD and coping with a heart attack. And, you are asked, did you think arnica is just a first aid remedy?

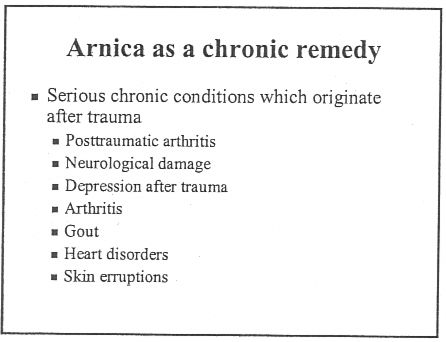
If that isn’t a list of "named diseases", what is? The code of ethics appears to be a total sham.
And of course never forget that the “arnica” doesn’t contain any arnica anyway. And if you don’t believe that you can read the words of Kate Chatfield, module leader on thie very course, as recorded in the minutes of evidence to the Select Committee on Science and Technology .
Q538 Lord Broers: I have a simple, technical question about homeopathy and drugs. Is it possible to distinguish between homeopathic drugs after they have been diluted? Is there any means of distinguishing one from the other?
Ms Chatfield: Only by the label.
You can read a lovely analysis of the views of Kate Chatfield by physicist A.P. Gaylard here.
Follow-up
The Daily Telegraph. January 8th 2009 Ian Douglas reported on this post: The workings of a BSc in homeopathy
The Lancashire Evening Post carried a big spread on January 15th, Professor seeks out the truth about ‘quackery’.
River’s Edge. News and thoughts from Preston, Lancashire reviewed the Lancashire Evening Post article on Saturday January 16th: Homeopathy at UCLAN, a degree in quackery.
The cost of trying to stop this material being revealed. UCLAN told me on 5 February 2010 that the legal costs alone were £80,307.94 (inc. VAT). That doesn’t include staff time and photocopying. I’m not convinced that this was a good way to spend taxpayers’ money.
More boring politics, but it matters. The two main recommendations of this Pittilo report are that
- Practitioners of Acupuncture, Herbal Medicine, Traditional Chinese Medicine should be subject to statutory regulation by the Health Professions Council
- Entry to the register should normally be through a Bachelor degree with Honours
For the background on this appalling report, see earlier posts.
A very bad report: gamma minus for the vice-chancellor
The Times (blame subeditor for the horrid title), and some follow up on the Times piece
The Health Professions Council breaks its own rules: the result is nonsense
Chinese medicine -acupuncture gobbledygook revealed
Consultation opens on the Pittilo report: help stop the Department of Health making a fool of itself
Why degrees in Chinese medicine are a danger to patients
The Department of Health consultation shuts on November 2nd. If you haven’t responded yet, please do. It would be an enormous setback for reason and common sense if the government were to give a stamp of official approval to people who are often no more than snake-oil salesman.
Today I emailed my submission to the Pittilo consultation to the Department of Health, at HRDListening@dh.gsi.gov.uk
The submission
I sent the following documents, updated versions of those already posted earlier.
- Submission to the Department of Health, for the consultation on the Pittilo report [download pdf].
- What is taught in degrees in herbal and traditional Chinese medicine? [download pdf]
- $2.5B Spent, No Alternative Med Cures [download pdf]
- An example of dangerous (and probably illegal) claims that are routinely made by TCM practitioners [download pdf]f
I also completed their questionnaire, despite its deficiencies. In case it is any help to anyone, this is what I said:
The questionnaire
Q1: What evidence is there of harm to the public currently as a result of the activities of acupuncturists, herbalists and traditional Chinese medicine practitioners? What is its likelihood and severity?
Harm
No Harm
Unsure
Comment
The major source of harm is the cruel deception involved in making false claims of benefit to desperate patients. This applies to all three.
In the case of herbal and TCM there is danger from toxicity because herbal preparations are unstandardised so those that do contain an active ingredient are given in an unknown dose. This is irresponsible and dangerous (but would not be changed by the proposals for regulation).
In addition TCM suffers from recurrent problems of contamination with heavy metals, prescription drugs and so on. Again this would not be the business of the proposed form of regulation.
Q2: Would this harm be lessened by statutory regulation? If so, how?
Yes
No
Unsure
The proposed form of regulation would be no help at all. The HPC has already said that it is not concerned with whether or not the drug works, and, by implication, does not see itself as preventing false health claims (just as the GCC doesn’t do this). False claims are the responsibility of Trading Standards who are meant to enforce the Consumer Protection Unfair Trading Regulations (May 2008), though they do not at present enforce them very effectively. Also Advertisng Standards. The proposed regulation would not help, and could easily hinder public safety as shown by the fact that the GCC has itself been referred to the Advertisng Standards Authority.
The questions of toxicity and contamination are already the responsibility of Trading Standards and the MHRA. Regulation by the HPC would not help at all. The HPC is not competent to deal with such questions.
Q3: What do you envisage would be the benefit to the public, to practitioners and to businesses, associated with introducing statutory regulation?
Significant benefit
Some benefit
No benefit
Unsure
This question is badly formulated because the answer is different according to whether you are referring to the public, to practitioners or to businesses.
The public would be endangered by the form of regulation that is proposed, as is shown very clearly by the documents that I have submitted separately.
In the case of practitioners and businesses, there might be a small benefit, if the statutory regulation gave the impression that HM and TCM had government endorsement and must therefore be safe and effective.
There is also one way that the regulation could harm practitioners and businesses. If the HPC received a very large number of complaints about false health claims, just as the GCC has done recently, not only would it cost a large amount of money to process the claims, but the attendant bad publicity could harm practitioners. It is quite likely that this would occur since false claims to benefit sick people are rife in the areas of acupuncture, HM and TCM.
Q4: What do you envisage would be the regulatory burden and financial costs to the public, to practitioners, and to businesses, associated with introducing statutory regulation? Are these costs justified by the benefits and are they proportionate to the risks? If so, in what way?
Justified
Not Justified
Unsure
Certainly not justified. Given that I believe that the proposed form of regulation would endanger patients, no cost at all would be justified. But even if there were a marginal benefit, the cost would be quite unjustified. The number of practitioners involved is very large. It would involve a huge expansion of an existing quango, at a time when the government is trying to reduce the number of quangos. Furthermore, if the HPC were flooded with complaints about false health claims, as the GCC has been, the costs in legal fees could be enormous.
Q5: If herbal and TCM practitioners are subject to statutory regulation, should the right to prepare and commission unlicensed herbal medicines be restricted to statutorily regulated practitioners?
Yes
No
Unsure
I don’t think it would make much difference. The same (often false) ideas are shared by all HM people and that would continue to be the same with or without SR.
Q6: If herbal and TCM practitioners are not statutorily regulated, how (if at all) should unlicensed herbal medicines prepared or commissioned by these practitioners be regulated?
They could carry on as now, but the money that would have been spent on SR should instead be used to give the Office of Trading Standards and the MHRA the ability to exert closer scrutiny and to enforce more effectively laws that already exist. Present laws, if enforced, are quite enough to protect the public.
Q7: What would be the effect on public, practitioners and businesses if, in order to comply with the requirements of European medicines legislation, practitioners were unable to supply manufactured unlicensed herbal medicines commissioned from a third party?
Significant effect
Some effect
No effect
Unsure
European laws,especialliy in food area, are getting quite strict about the matters of efficacy. The proposed regulation, which ignores efficacy, could well be incompatible with European law, if not now, then soon. This would do no harm to legitimate business though it might affect adversely businesses which make false claims (and there are rather a lot of the latter).
Q8: How might the risk of harm to the public be reduced other than by orthodox statutory regulation? For example by voluntary self-regulation underpinned by consumer protection legislation and by greater public awareness, by accreditation of voluntary registration bodies, or by a statutory or voluntary licensing regime?
Voluntary self-regulation
Accreditation of voluntary bodies
Statutory or voluntary licensing
Unsure
I disagree with the premise, for reasons given in detail in separate documents. I believe that ‘orthodox statutory regulation’, if that means the Pittilo proposals, would increase, not decrease, the risk to the public. Strengthening the powers of Trading Standards, the MHRA and such consumer protection legislation would be far more effective in reducing risk to the public than the HPC could ever be. Greater public awareness of the weakness of the evidence for the efficacy of these treatments would obviously help too, but can’t do the job on its own.
Q10: What would you envisage would be the benefits to the public, to practitioners, and to businesses, for the alternatives to statutory regulation outlined at Question 8?
It depends on which alternative you are referring to. The major benefit of enforcement of existing laws by Trading Standards and/or the MHRA would be (a) to protect the public from risk, (b) to protect the public from health fraud and (c) almost certainly lower cost to the tax payer.
Q11: If you feel that not all three practitioner groups justify statutory regulation, which group(s) does/do not and please give your reasons why/why not?
Acupuncture
Herbal Medicine
TCM
Unsure
None of them. The differences are marginal. In the case of acupuncture there has been far more good research than for HM or TCM. But the result of that research is to show that in most cases the effects are likely to be no more than those expected of a rather theatrical placebo. Furthermore the extent to which acupuncture has a bigger effect than no-acupuncture in a NON-BLIND comparison, is usually too small and transient to offer any clinical advantage (so it doesn’t really matter whether the effect is placebo or not, it is too small to be useful).
In the case of HM, and even more of TCM, there is simply not enough research to give much idea of their usefulness, with a small handful of exceptions.
This leads to a conclusion that DH seems to have ignored in the past. It makes absolutely no sense to talk about “properly trained practitioners” without first deciding whether the treatments work or not. There can be no such thing as “proper training” in a discipline that offers no benefit over placebo. It is a major fault of the Pittilo recommendations that they (a) ignore this basic principle and (b) are very over-optimistic about the state of the evidence.
Q12: Would it be helpful to the public for these practitioners to be regulated in a way which differentiates them from the regulatory regime for mainstream professions publicly perceived as having an evidence base of clinical effectiveness? If so, why? If not, why not?
Yes
No
Unsure
It might indeed be useful if regulation pointed out the very thin evidence base for HM and TCM but it would look rather silly. The public would say how can it be that the DH is granting statutory regulation to things that don’t work?
Q13: Given the Government’s commitment to reducing the overall burden of unnecessary statutory regulation, can you suggest which areas of healthcare practice present sufficiently low risk so that they could be regulated in a different, less burdensome way or de-regulated, if a decision is made to statutorily regulate acupuncturists, herbalists and traditional Chinese medicine practitioners?
Yes
No
Unsure
As stated above, the.only form of regulation that is needed, and the only form that would protect the public, is through consumer protection regulations, most of which already exist (though they are enforced in a very inconsistent way). Most statutory regulation is objectionable, not on libertarian grounds, but because it doesn’t achieve the desired ends (and is expensive). In this case of folk medicine, like HM and TCM, the effect would be exactly the opposite of that desired as shown in separate documents that I have submitted to the consultation.
Q14: If there were to be statutory regulation, should the Health Professions Council (HPC) regulate all three professions? If not, which one(s) should the HPC not regulate?
Yes
No
Unsure
The HPC should regulate none of them. It has never before regulated any form of alternative medicine and it is ill-equipped to do so. Its statement that it doesn’t matter that there is very little evidence that the treatments work poses a danger to patients (as well as being contrary to its own rules).
Q15: If there were to be statutory regulation, should the Health Professions Council or the General Pharmaceutical Council/Pharmaceutical Society of Northern Ireland regulate herbal medicine and traditional Chinese medicine practitioners?
HPC
GPC/PSNI
Unsure
Neither. The GPC is unlikely to care about whether the treatments work any more than the RPSGB did, or the GCC does now. The problems would be exactly the same whichever body did it.
Q16: If neither, who should and why?
As I have said repeatedly, it should be left to Trading Standards, the MHRA and other consumer protection regulation.
Q17:
a) Should acupuncture be subject to a different form of regulation from that for herbalism and traditional Chinese medicine? If so, what?
Yes
No
Unsure
b) Can acupuncture be adequately regulated through local means, for example through Health and Safety legislation, Trading Standards legislation and Local Authority licensing?
Yes
No
Unsure
(a) No -all should be treated the same. Acupuncture is part of TCM
(b) Yes
Q18.
a) Should the titles acupuncturist, herbalist and [traditional] Chinese medicine practitioner be protected?
b) If your answer is no which ones do you consider should not be legally protected?
Yes
No
Unsure
No. It makes no sense to protect titles until such time as it has been shown that the practitioners can make a useful contribution to medicine (above placebo effect). That does not deny that placebos may be useful at times. but if that is all they are doing, the title should be ‘placebo practitioners’.
Q19: Should a new model of regulation be tested where it is the functions of acupuncture, herbal medicine and TCM that are protected, rather than the titles of acupuncturist, herbalist or Chinese medicine practitioner?
Yes
No
Unsure
No. This makes absolutely no sense when there is so little knowledge about what is meant by the ” functions of acupuncture, herbal medicine and TCM”.Insofar as they don’t work (better than placebo), there IS no function. Any attempt to define function when there is so little solid evidence (at least for HM and TCM) is doomed to failure.
Q20: If statutory professional self-regulation is progressed, with a model of protection of title, do you agree with the proposals for “grandparenting” set out in the Pittilo report?
Yes
No
Unsure
No. I believe the Pittilo report should be ignored entirely. The whole process needs to be thought out again in a more rational way.
Q22: Could practitioners demonstrate compliance with regulatory requirements and communicate effectively with regulators, the public and other healthcare professionals if they do not achieve the standard of English language competence normally required for UK registration? What additional costs would occur for both practitioners and regulatory authorities in this case?
Yes
No
Unsure
No. It is a serious problem, in TCM especially, that many High Street practitioners speak hardly any English at all. That adds severely to the already considerable risks. There would be no reliable way to convey what was expected of them. it would be absurd for the taxpayer to pay for them to learn English for the purposes of practising TCM (of course there might be the same case as for any other immigrant for teaching English on social grounds).
Q23: What would the impact be on the public, practitioners and businesses (financial and regulatory burden) if practitioners unable to achieve an English language IELTS score of 6.5 or above are unable to register in the UK?
Significant impact
Some impact
No impact
Unsure
The question is not relevant. The aim of regulation is to protect the public from risk (and it should be, but isn’t, an aim to protect them from health fraud). It is not the job of regulation to promote businesses
Q24: Are there any other matters you wish to draw to our attention?
I have submitted three documents via HRDListening@dh.gsi.gov.uk. The first of these puts the case against the form of regulation proposed by Pittilo, far more fluently than is possible in a questionnaire.
Another shows examples of what is actually taught in degrees in acupuncture, HM and TCM. They show very graphically the extent to which the Pittilo proposals would endanger the public, if they were to be implemented..
The first post was NICE falls for Bait and Switch by acupuncturists and chiropractors: it has let down the public and itself.
That was followed by NICE fiasco, part 2. Rawlins should withdraw guidance and start again.
Since then, something of a maelstrom has engulfed NICE, so it’s time for an update.
It isn’t only those who are appalled that NHS should endorse voodoo medicine on the basis of very slim evidence who are asking NICE to rethink their guidance on low back pain. Pain specialists are up in arms too, and have even started a blog, ‘Not Nearly as NICE as you think …‘, to express their views. Equally adverse opinions are being expressed in the Britsh Medical Journal. A letter there is signed by over 50 specialists in pain medicine. It ends thus
“Because of these new guidelines patients will continue to experience unnecessary pain and suffering and their rights to appropriately individually tailored treatment have been removed on the basis of a flawed analysis of available evidence. We believe the guidelines do not reflect best practice, remove patient choice and are not in our patients’ best interests.”
In a contribution headed “NICE misguidance”. Dr Michel Vagg ends
It seems to me that this guideline has been used as a propaganda vehicle to allow cherry-picked evidence to be enshrined as doctrine. This is an abuse of the guideline development process . . . ”
I have to say, though, that it seems to me that some of these people are promoting their own interests as much as chiropractors and acupuncturists. The evidence that spinal injections produce worthwhile benefits seems to be as thin as the evidence that chiropractic and acupuncture produce worthwhile benefits. But no doubt the injections are good for the budgets of PCTs or private practice doctors.. Could it perhaps be the case that some of the clinicians’ anger is being generated by doctors who are rushing to defend their own favourite ineffective treatment?
Why, oh why, can’t either NICE or the pain consultants bring themselves to state the obvious, that nothing works very well. The only thing that can be said for most of the regular treatments is that although they may not be much more effective than acupuncture or chiropractic, at least they don’t come with the intellectually-offensive hokum that accompanies the latter. Very sensible attempts have been made to identify the cause of low back pain [reviewed here], Occasionally they succeed. Mostly they don’t.
One clinician’s letter deserves special attention because it goes into the evidence, and the costs, in some detail. Its conclusions are very different from those in the NICE guidance.
The letter, a Review of NICE guidance, is from Dr C.J.D. Wells [download the whole letter]. He is a pain relief consultant from Liverpool.
Let’s look at some highlights.
Wells points out the absurdity of the cost estimates
“In the pricing section, they estimate that this will require an increase of facilities so that 3,500 patients can be treated instead of 1,000 at present (again see comments on pricing). This is not many treatments for the 20 million sufferers, of whom we can estimate that at least 2 million will have significant long-term disability and psychological distress”
And that is without even costing all the secondary costs of miseducating a new generation of students in fables about “Qi”, meridians, energy flow, subluxations and innate intelligence.
“The abysmal ignorance of the committee is reflected in the poor overall advice. So if you have a committee with special interests in Exercise, Manipulation, PMP’s, and Surgery, and you call an expert on Acupuncture, you get advice to use Exercise, Manipulation, Acupuncture, PMP’s and Surgery. Amazing.”
Another pain consutant, Charles Guaci, says in a comment in the Daily Mail.
I am a Pain Consultant of 30 years experience, have published two books (one translated into different languages).
NICE never asked me for my opinion.
This is the most ridicuculous pseudo-scientific document I have ever seen.
The panel consisted of a surgeon, psychologist, osteopath, acupuncturist a physiotherapist and an academic; not one pain consultant! The conclusions are simply a means of increasing the employment of their friends!
All evidence submitted to NICE was ignored.
It is almost certain than unless NICE rethink their ideas that Pain Consultants will be seeking a judicial review as well as full disclosure of how the panel arrived at their bizarre findings under the Freedom of information act.
Patients should realise that they are being taken for a ride.
Despite the outcry from opponents of magic medicine and from pain specialists, the assessment by the normally excellent NHS Choices site was disappointing. It made no mention at all of the secondary consequences of recommending CAM and described the assertions of the guidance group quite uncritically.
The reputation of NICE
NICE has been criticised before, though usually unjustly. In the past I have often supported them. For example. when NICE said that treatment of dementia with anticholinesterase drugs like galantamine was ineffective, there was a great outcry, but NICE were quite right. There is little or no rationale for such treatments, and more importantly, very little evidence that they work. But patients, especially when they are desperate, have greater faith in drug treatments than most pharmacologists, They want to clutch at straws. A bit like the NICE guidance committee, faced with a bunch of treatments most of which are almost ineffective, clutched at the straws of acupuncture and chiropractic. But this time it isn’t only the patients who are cross. It is most of the medical and scientific world too.
One interpretation of these bizarre events is that they represent a case of medical/scientific arrogance. Ben Goldacre wrote of another aspect of the same problem thus week, in Dodgy academic PR [download the paper on which this is based].
The first job of a scientist is to say openly when the answer to a question is not known. But scientists are under constant pressure to exaggerate the importance of their results. Last year we published an article which I feel may, if verified, turn out to be the second most important that I have ever been an author on. Because it happened to be published in Nature (not because of its quality), a press release was written (by an arts graduate!). It took some argument to prevent the distorted and exaggerated account being imposed on the public. This is typical of the sort of thing reported in Goldacre’s column. I reported a similar case a while ago, Why honey isn’t a wonder cough cure: more academic spin.
If NICE does not reconsider this guidance, it is hard to see how it can be taken seriously in the future. I hope that when NICE’s director, Professor Sir Michael Rawlins, returns from his trips abroad, he will find time to look at the case himself.
Indirectly, then, it can be argued that NICE’s bizarre guidance is just another manifestation of the management of science being passed from the hands of scientists into the hands of administrators and spin experts. It is yet another example of DC’s rule
Never trust anyone who uses the word ‘stakeholder’
Some bone-headed bureaucrat decides that any charlatan or quack is a ‘stakeholder’ in the provision of NHS care and gives them a quite disproportionate say in how taxpayers’ money is spent. The bureaucrats are so busy following processes and procedures, ticking boxes, and so deficient in scientific education, that they failed to notice that they’ve been caught out by the old trick of used car salesmen, bait and switch.
The consequences
The expected consequences have already started to materialise. The Prince of Wales’ Foundation for Magic Medicine is jubilant about having been endorsed by NICE. And I’m told that “The chiropractors have now just written letters to all health boards in Scotland asking for contracts for their services to deal with back pain”.
There could hardly have been a worse time for NICE to endorse chiropractic. We are in the middle of a storm about free speech because of the disgraceful action of the British Chiropractic Associaton in suing one of our best science writers, Simon Singh, for defamation because he had the temerity to express an opinion, Of course, even if the BCA wins in court, it will be the overall loser, because chiropractic claims are now being scrutinised as never before (just look at what they told me).
Follow-up
A much-cited paper. The paper that is most often cited by chiropractors who claim to be able to cure colic by spinal manipulation is Klougart N, Nilsson N and Jacobsen J (1989) Infantile Colic Treated by Chiropractors: A Prospective Study of 316 Cases, J Manip Physiol Ther,12:281-288. This is not easy to get hold of but Steve Vogel has sent me s scanned copy which you can download here. As evidence it is about as useless as the infamous Spence study so beloved of homeopaths. There was no control group at all. It simply follows 316 babies and found that most of them eventually got better. Well, they do, don’t they? It is a sign of the pathetic standard of reaearch in chiropractic that anyone should think this paper worth mentioning at all.
June 6 2009. More flak for NICE from the Royal College of Anaesthetists, and more adverse comment in the BMJ. And of course the blogs. for example, “If this is “evidence based medicine” I want my old job back“.
“Acupuncture on the NHS: a dangerous precedent”: a good analysis at counterknowledge.com.
June 6 2009, Comment sent to the BMJ. The comment was submitted, as below, early on Friday 5th June. The BMJ said it was a “sensitive issue” and for the next five days lawyers pondered over it.
Underwood and Littlejohns describe their guidance as being a “landmark”. I can only agree with that description. It is the first time that NICE has ever endorsed alternative medicine in the face of all the evidence. The guidance group could hardly have picked a worse moment to endorse chiropractic. Chiropractors find it so hard to find evidence for their practices that, when one of our finest science writers, Simon Singh, asked to see the evidence they sued him for defamation. I suggest that the guidance group should look at the formidable list of people who are supporting Singh, after his brave decision to appeal against this iniquitous persecution.
Of course I’m sure this bizarre decision has nothing to do with the presence on the guidance group of Peter Dixon, chair of the General Chiropractic Council. Nevertheless, I am curious to know why it is that when I telephoned two of the practices belonging to Peter Dixon Associates, I was told that they could probably treat infantile colic and asthma. Such claims have just been condemned by the Advertising Standards Authority.
The low back pain guidance stands a good chance of destroying NICE’s previously excellent reputation for dispassionate assessment of benefits and costs. Yes, that is indeed a landmark of sorts.
If NICE is ever to recover its reputation, I think that it will have to start again. Next time it will have to admit openly that none of the treatments work very well in most cases. And it will have to recognise properly the disastrous cultural consequences of giving endorsement to people who, when asked to produce evidence, resort to legal intimidation.
Eventually, on Wednesday 10 June the comment appeared in the BMJ, and it wasn’t greatly changed. Nevertheless if is yet another example of legal chill. This is the final version.
Underwood and Littlejohns describe their guidance as being a “landmark”. I can only agree with that description. It is the first time that NICE has ever endorsed alternative medicine in the face of all the evidence. The guidance group could hardly have picked a worse moment to endorse chiropractic. Chiropractors are so sensitive about criticisms of their practices that, when one of our finest science writers, Simon Singh, queried the evidence-base for their therapeutic claims they sued him for defamation. I suggest that the guidance group should look at the formidable list of people who are supporting Singh, after his brave decision to appeal against an illiberal court ruling in this iniquitous persecution.
One wonders whether this bizarre decision by NICE has anything to do with the presence on the guidance group of Peter Dixon, chair of the General Chiropractic Council. I am also curious to know why it is that when I telephoned two of the practices belonging to Peter Dixon Associates, I was told that chiropractic could be effective in the treatment of infantile colic and asthma. Similar claims about treating colic have just been condemned by the Advertising Standards Authority.
The low back pain guidance stands a good chance of destroying NICE’s previously excellent reputation for dispassionate assessment of benefits and costs. Yes, that is indeed a landmark of sorts.
If NICE is ever to recover its reputation, I think that it will have to start again. Next time it will have to admit openly that none of the treatments works very well in most cases. And it will have to recognise properly the disastrous cultural consequences of giving endorsement to people who, instead of engaging in scientific debate, resort to legal intimidation.
Bait and switch. Oh dear, oh dear. Just look at this. British Chiropractic Association tell their members to hide their sins from prying eyes.
Excellent round-up of the recent outburst of writing about “chiroquacktic” (Tut, tut, is there no respect?).
Dr Crippen writes “NICE recommends a cure for all known disease” [Ed some exaggeration, surely]
In March 2007 I wrote a piece in Nature on Science degrees without the science. At that time there were five “BSc” degrees in homeopathy. A couple of weeks ago I checked the UCAS site for start in 2009, and found there was only one full “BSc (hons)” left and that was at Westminster University.
Today I checked again and NOW THERE ARE NONE.
A phone call to the University of Westminster tonight confirmed that they have suspended entry to their BSc (Hons) homeopathy degree.
They say that they have done so because of “poor recruitment”. It was a purely financial decision. Nothing to do with embarrasment. Gratifying though it is that recruits for the course are vanishing, that statement is actually pretty appalling It says that the University of Westminster doesn’t care whether it’s nonsense, but only about whether it makes money.
Nevertheless the first part of this post is not entirely outdated before it even appeared, because homeopathy will still be taught as part of Complementary Therapies. And Naturopathy and “Nutritional Therapy” are still there..
According to their ‘School of Integrated Health‘, “The University of Westminster has a vision of health care for the 21st Century”. Yes, but it is what most people would call a vision of health care in the 18th century.
The revelation that the University of Westminster teaches that Amethysts emit high Yin energy caused something of a scandal.
Since then I have acquired from several sources quite a lot more of their teaching material, despite the fact that the university has refused to comply with the Freedom of Information Act.
In view of the rather silly internal review conducted by Westminster’s Vice-Chancellor, Professor Geoffrey Petts, this seems like a good moment to make a bit more of it public,
I think that revelation of the material is justified because it is in the
public interest to know how the University if Westminster is spending taxpayers’ money. Another motive is to defend the reputation of the post-1992 universities. I have every sympathy with the ex-polytechnics in their efforts to convert themselves into universities. In many ways they have succeeded. That effort
is impeded by teaching mystical versions of medicine.
If the University of Westminster is being brought into disrepute, blame its vice-chancellor, not me.
Homeopathic spiders
Here are a few slides from a lecture on how good spider venom is for you. It is from Course 3CTH502 Homeopathic Materia Medica II. No need to worry though, because they are talking about homeopathic spider venom, so there is nothing but sugar in the pills. The involvement of spiders is pure imagination. No more than mystical gobbledygook.


You are in hurry, or play with your fingers? You need spider venom pills (that contain no spider venom).




You break furniture? Time goes too fast for you? Try the tarantula-free tarantula pills.


You are preoccupied with sex? You play with ropes? What you need is Mygale (which contains no Mygale)


Much more seriously, the same sugar pills are recommended for serious conditions, chorea, ‘dim sight’, gonorrhoea, syphilis and burning hot urine.

This isn’t just preposterous made-up stuff. It is dangerous.
There is a whole lot more fantasy stuff in the handouts for Homeopathy Materia Medica II (3CTH502). Here are a couple of examples.
Aurum metallicum (metallic gold) [Download the whole handout]
Affinities MIND, VASCULAR SYSTEM, Nerves, Heart, Bones, Glands, Liver, Kidneys, RIGHT SIDE, Left side.
Causations Emotions. Ailments from disappointed love and grief, offence or unusual responsibility, abuse of mercury or allopathic drugs.
Aurum belongs to the syphilitic miasm but has elements of sycosis (Aur-Mur).
Potassium salts are the subject of some fine fantasy, in “The Kali’s” [sic]. (there is much more serious stuff to worry about here than a few misplaced apostrophes.). [Download the whole handout]
“The radioactive element of potassium emits negative electrons from the atom nucleus and is thought to be significant in the sphere of cell processes especially in relation to functions relating to automatism and rhythmicity.”
“Kali people are very conscientious with strong principles. They have their rules and they stick to them, ‘a man of his word’.”
“Potassium acts in a parasympathetic way, tending towards depression”
“They [“Kali people=] are not melancholic like the Natrum’s but rather optimistic.”
Radioactive potassium is involved in automaticity? Total nonsense.
Where is the science?
Yes, it is true that the students get a bit of real science. There isn’t the slightest trace that I can find of any attempt to resolve the obvious fact that what they are taught in the science bits contradict directly what they are told in the other bits. Sounds like a recipe for stress to me.
They even get a bit of incredibly elementary statistics. But they can’t even get that right. This slide is from PPP – Res Quant data analysis.
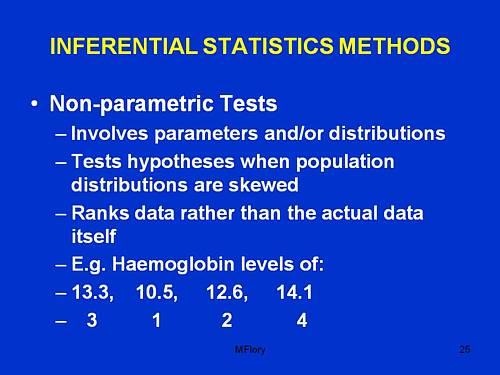
“Involves parameters and/or distributions”. This has no useful meaning whatsoever, that I can detect.
“Tests hypotheses when population distributions are skewed”. Well yes, though nothing there about forms of non-Gaussian properties other than skew, nothing about normalising transformations, and nothing about the Central Limit theorem.
“Ranks data rather than the actual data itself”. This is plain wrong. Randomisation tests on the original data are generally the best (uniformly most powerful) sort of non-parametric test. It seems to have escaped the attention of the tutor that ranking is a short-cut approximation that allowed tables to be constructed, before we had computers.
The students are told about randomised controlled trials. But amazingly in the lecture PPP-RCTs, the little matter of blinding is barely mantioned. And the teacher’s ideas about randomisation are a bit odd too.

Sorry, but if you fiddle the randomisation, no amount of “careful scrutiny” will rescue you from bias.
An Introduction to Naturopathic Philosophy
Naturopathy is just about as barmy as homeopathy. You can see something about it at the University of Wales. How about this slide from Westminster’s An Introduction to Naturopathic Philosophy.

So if you get tuberculosis, it isn’t caused by Mycobacterium tuberculosis? And the symptoms are “constructive”? So you don;t need to do anything. It’s all for the best really.
This isn’t just nonsense. It’s dangerous nonsense.
Traditional Chinese Medicine
Ever wondered what the mysterious “Qi” is? Worry no more. All is explained on this slide.

It means breath, air, vapour, gas, energy, vitalism. Or perhaps prana? Is that quite clear now?

What can we make of this one? Anyone can see that the description is barely written in English and that vital information is missing (such as the age of the woman). And it’s nonsense to suggest that “invasion of cold” (during keyhole surgery!) would cause prolonged constriction of blood vessels (never mind that it would “consume yang qi”). Not being a clinician, I showed it to an oncologist friend. He said that it was impossible to tell from the description whether the problem was serious or not, but that any abdominal pain should be investigated properly. There isn’t anything here about referral for proper investigation. Just a lot of stuff about ginger and cinnamon. Anyone who was taught in this way could be a real danger to the public. It isn’t harmless nonsense It’s potentially harmful nonsense.
And finally, it’s DETOX
Surely everyone knows by now that ‘detox’ is no more than a marketing word? Well not at the University of Westminster. They have a long handout that tells you all the usual myths and a few new ones.
It is written by Jennifer Harper-Deacon, who describes herself modestly, thus.
Jennifer Harper-Deacon is a qualified and registered Naturopath and acupuncturist who holds a PhD in Natural Health and MSc in Complementary Therapies. She is a gifted healer and Reiki Master who runs her own clinic in Surrey where she believes in treating the ‘whole’ person by using a combination of Chinese medicine and naturopathic techniques that she has qualified in, including nutritional medicine, Chinese and Western herbalism, homoeopathy, applied kinesiology, reflexology, therapeutic massage, aromatherapy and flower remedies.
It seems that there is no limit on the number of (mutually incompatible) forms of nuttiness that she believes. Here are a few quotations from her handout for Westminster students.
“Detoxification is the single most powerful tool used by natural health professionals to prevent and reverse disease”
What? To “prevent and reverse” malaria? tuberculosis? Parkinson’s disease? AIDS? cancer?
“When you go on to a raw food only diet, especially fruit, the stored toxins are brought up from the deep organs such as the liver and kidneys, to the superficial systems of elimination.”;
Very odd. I always though that kidneys were a system of elimination.
“The over-use and mis-use of antibiotics has weakened the body’s ability to attack and destroy new strains of resistant bacteria, virulent viruses, which have led to our immune system becoming compromised.”
Certainly over-use and mis-use are problems. But I always thought it was the bacteria that became resistant.
“The beauty about detoxification therapy is that it addresses the very causative issues of health problems”
That is another dangerous and silly myth. Tuberculosis is not caused by mythical and un-named “toxins”. It is caused by Mycobacterium tuberculosis.
“Naturopathy follows the logic of cause and effect therefore believes that we simply need pure food and water, sunshine, air, adequate rest and sleep coupled with the right amount of exercise for health.”
Try telling that to someone with AIDS.
“Colon cleansing is one of the most important parts of any detoxification programme.”
The strange obsession with enemas in the alternative world is always baffling.
“Frankincense: holds the capacity to physically strengthen our defence system and can rebuild energy levels when our immune system is weak. Revered as a herb of protection, frankincense can also strengthen our spiritual defences when our Wei qi is low, making us more susceptible to negative energies. This calming oil has the ability to deepen the breath, helping us to let go of stale air and emotions, making it ideal oil to use inhale prior to meditating.”
This is so much hot air. There is a bit of evidence that frankincense might have some anti-inflammatory action and that’s it.
But this has to be my favourite.
“Remember when shopping to favour fruits and vegetables which are in season and locally grown (and ideally organic) as they are more vibrationally compatible with the body.”
Locally grown vegetables are “more vibrationally compatible with the body”? Pure mystical gobbledygook. Words fail me.
OK there’s a whole lot more, but that will do for now.
It’s good that Westminster is shutting down its Homeopathy BSc, but it seems they have a bit further to go.
Recently I wrote a piece for the National Health Executive (“the Independent Journal for Senior Health Service Managers”), with the title Medicines that contain no medicine and other follies
In the interests of what journalists call balance (but might better be called equal time for the Flat Earth Society), an article appeared straight after mine, Integrating Homeopathy into Primary Care. It was by Rachel Roberts “Research consultant for the Society of Homeopaths”.
This defence was so appalling that I sent them a response (after first doing a bit of checking on its author). To my surprise, they published the response in full [download pdf of printed version]. Their title was

As always, the first step is to Google the author, to find out a bit more. It seems that Rachel Roberts runs a business Integrated Homeopathic Training. (a financial interest that was not mentioned in her article). She will sell you flash cards (‘Matmedcards’) for £70 (+£9 p&p) for 120 cards (yes, seriously). The card for Conium maculatum is remarkable. It says on the reverse side

Yes, it says (my emphasis)
“The poison used to execute Socrates. No 1 remedy for scirrhous breast cancer. Esp after blow to the breast”
No doubt she would claim that the word “remedy” was a special weasel word of homeopaths that did not imply any therapeutic efficacy. But its use in this context seems to me to be cruel deception, even murderous. It also appears to breach the Cancer Act 1939, as well as the Unfair Trading laws.
I asked the Bristol Trading Standards Office, and got a reply remarkably quickly. It ended thus.
“. . . . the use of the card for “hemlock” as an example amounts to advice in connection with the treatment (of cancer)”. I will initially write to IHT and require that they remove this, and any other, reference to cancer treatment from their website.
When I checked again a couple of weeks later, the hemlock sample card had been been replaced by one about chamomile (it is described as the opium of homeopathy. Luckily the pills contain no opium (and no chamomile either) or that would be breaking another law. Bafflingly, it is not (yet) against the law to sell pills that contain no trace of the ingredient on the label, if they are labelled ‘homeopathic’.
Presumably the packs still contain a claim to cure cancer. And what is said in the privacy of the consulting room will never be known.
Political correctness is a curious thing. I felt slightly guilty when I reported this breach of the Cancer Act. It felt almost sneaky. The feeling didn’t last long though. We are talking about sick people here.
It isn’t hard to imagine a desperate woman suffering from cancer reading that Ms Roberts knows the “No 1 remedy for scirrhous breast cancer”. She might actually believe it. She might buy some hemlock pills that contain no hemlock (or anything else). She might die as a result. It is not a joke. It is, literally, deadly serious.
It is also deadly serious that the Department of Health and some NHS managers are so stifled by political correctness that they refer to homeopaths as “professionals” and pay them money.
Ms Roberts, in her article, is at pains to point out that
|
 |
Well it is already well known that the the Code of Ethics of the Society of Homeopaths is something of a joke. This is just one more example.
The Code of Ethics, para 72 says homeopaths have a legal obligation
“To avoid making claims (whether explicit or implied; orally or in writing) implying cure of any named disease.”
Like, perhaps, claiming to have the “No 1 remedy for scirrhous breast cancer”?
Obviously voluntary self-regulation isn’t worth the paper it’s written on.
You don’t need to go to her web site to find “claims . . . implying cure of any named disease”. In her article she says
“Conditions which responded well to homeopathy included childhood eczema and asthma, migraine, menopausal problems, inflammatory bowel disease, irritable bowel syndrome, arthritis, depression and chronic fatigue syndrome.”
No doubt they will say that the claim that asthma and migraine “responded well” to their sugar pills carries no suggestion that they can cure a named disease. And if you believe that, you’ll believe anything.
I have to say that I find Ms Robert’s article exceedingly puzzling. It comes with 29 references, so it looks, to use Goldacre’s word, ‘sciencey’. If you read the references, and more importantly, know about all the work that isn’t referred to, you see it is the very opposite of science. I see only two options.
Either it is deliberate deception designed to make money, or it shows, to a mind-boggling extent, an inability to understand what constitutes evidence.
The latter, more charitable, view is supported by the fact that Ms Roberts trots out, yet again, the infamous 2005 Spence paper, as though it constituted evidence for anything at all. In this paper 6544 patients at the Bristol Homeopathic Hospital were asked if the felt better after attending the out-patient department. Half of them reported that the felt ‘better’ or ‘much better’. Another 20% said they were ‘slightly better’ (but that is what you say to be nice to the doctor). The patients were not compared with any other group at all. What could be less surprising than that half of the relatively minor complaints that get referred for homeopathy get ‘better or much better’ on their own?
This sort of study can’t even tell you if homeopathic treatment has a placebo effect, never mind that it has a real effect of its own. It is a sign of the desperation of homeopaths that they keep citing this work.
Whatever the reason, the conclusion is clear. Never seek advice from someone who has a financial interest in the outcome. Ms Roberts makes her living from homeopathy. If she were to come to the same conclusion as the rest of the world, that it is a placebo and a fraud, her income would vanish. It is asking too much of anyone to do that.
| This is the mistake made time and time again by the Department of Health and by the NHS. The Pittilo report does the same thing The execrably bad assessment of evidence in that report is, one suspects, not unrelated to the fact that it was done entirely by people who would lose their jobs if they were to come to any conclusion other than their treatments work. |  |
At present , the regulation of alternative medicine is chaotic because the government and the dozen or so different quangos involved are trying to regulate while avoiding the single most important question – do the treatments work?
They should now grasp that nettle and refer the question to NICE.
Follow-up
I’m perfectly happy to think of alternative medicine as being a voluntary, self-imposed tax on the gullible (to paraphrase Goldacre again). But only as long as its practitioners do no harm and only as long as they obey the law of the land. Only too often, though, they do neither.
When I talk about law, I don’t mean lawsuits for defamation. Defamation suits are what homeopaths and chiropractors like to use to silence critics. heaven knows, I’ve becomes accustomed to being defamed by people who are, in my view. fraudsters, but lawsuits are not the way to deal with it.

I’m talking about the Trading Standards laws Everyone has to obey them, and in May 2008 the law changed in a way that puts the whole health fraud industry in jeopardy.
The gist of the matter is that it is now illegal to claim that a product will benefit your health if you can’t produce evidence to justify the claim.
I’m not a lawyer, but with the help of two lawyers and a trading standards officer I’ve attempted a summary. The machinery for enforcing the law does not yet work well, but when it does, there should be some very interesting cases.
The obvious targets are homeopaths who claim to cure malaria and AIDS, and traditional Chinese Medicine people who claim to cure cancer.
But there are some less obvious targets for prosecution too. Here is a selection of possibilities to savour..
- Universities such as Westminster, Central Lancashire and the rest, which promote the spreading of false health claims
- Hospitals, like the Royal London Homeopathic Hospital, that treat patients with mistletoe and marigold paste. Can they produce any real evidence that they work?
- Edexcel, which sets examinations in alternative medicine (and charges for them)
- Ofsted and the QCA which validate these exams
- Skills for Health and a whole maze of other unelected and unaccountable quangos which offer “national occupational standards” in everything from distant healing to hot stone therapy, thereby giving official sanction to all manner of treatments for which no plausible evidence can be offered.
- The Prince of Wales Foundation for Integrated Health, which notoriously offers health advice for which it cannot produce good evidence
- Perhaps even the Department of Health itself, which notoriously referred to “psychic surgery” as a profession, and which has consistently refused to refer dubious therapies to NICE for assessment.
The law, insofar as I’ve understood it, is probably such that only the first three or four of these have sufficient commercial elements for there to be any chance of a successful prosecution. That is something that will eventually have to be argued in court.
But lecanardnoir points out in his comment below that The Prince of Wales is intending to sell herbal concoctions, so perhaps he could end up in court too.
The laws
We are talking about The Consumer Protection from Unfair Trading Regulations 2008. The regulations came into force on 26 May 2008. The full regulations can be seen here, or download pdf file. They can be seen also on the UK Statute Law Database.
The Office of Fair Trading, and Department for Business, Enterprise & Regulatory Reform (BERR) published Guidance on the Consumer Protection from Unfair Trading Regulations 2008 (pdf file),
Statement of consumer protection enforcement principles (pdf file), and
The Consumer Protection from Unfair Trading Regulations: a basic guide for business (pdf file).
Has The UK Quietly Outlawed “Alternative” Medicine?
On 26 September 2008, Mondaq Business Briefing published this article by a Glasgow lawyer, Douglas McLachlan. (Oddly enough, this article was reproduced on the National Center for Homeopathy web site.)
“Proponents of the myriad of forms of alternative medicine argue that it is in some way “outside science” or that “science doesn’t understand why it works”. Critical thinking scientists disagree. The best available scientific data shows that alternative medicine simply doesn’t work, they say: studies repeatedly show that the effect of some of these alternative medical therapies is indistinguishable from the well documented, but very strange “placebo effect” ”
“Enter The Consumer Protection from Unfair Trading Regulations 2008(the “Regulations”). The Regulations came into force on 26 May 2008 to surprisingly little fanfare, despite the fact they represent the most extensive modernisation and simplification of the consumer protection framework for 20 years.”
The Regulations prohibit unfair commercial practices between traders and consumers through five prohibitions:-
- General Prohibition on Unfair Commercial
Practices (Regulation 3)- Prohibition on Misleading Actions (Regulations 5)
- Prohibition on Misleading Omissions (Regulation 6)
- Prohibition on Aggressive Commercial Practices (Regulation 7)
- Prohibition on 31 Specific Commercial Practices that are in all Circumstances Unfair (Schedule 1). One of the 31 commercial practices which are in all circumstances considered unfair is “falsely claiming that a product is able to cure illnesses, dysfunction or malformations”. The definition of “product” in the Regulations includes services, so it does appear that all forms medical products and treatments will be covered.
Just look at that!
| One of the 31 commercial practices which are in all circumstances considered unfair is “falsely claiming that a product is able to cure illnesses, dysfunction or malformations” |
Section 5 is equally powerful, and also does not contain the contentious word “cure” (see note below)
Misleading actions
5.—(1) A commercial practice is a misleading action if it satisfies the conditions in either paragraph (2) or paragraph (3).
(2) A commercial practice satisfies the conditions of this paragraph—
(a) if it contains false information and is therefore untruthful in relation to any of the matters in paragraph (4) or if it or its overall presentation in any way deceives or is likely to deceive the average consumer in relation to any of the matters in that paragraph, even if the information is factually correct; and
(b) it causes or is likely to cause the average consumer to take a transactional decision he would not have taken otherwise.
These laws are very powerful in principle, But there are two complications in practice.
One complication concerns the extent to which the onus has been moved on to the seller to prove the claims are true, rather than the accuser having to prove they are false. That is a lot more favourable to the accuser than before, but it’s complicated.
The other complication concerns enforcement of the new laws, and at the moment that is bad.
Who has to prove what?
That is still not entirely clear. McLachlan says
“If we accept that mainstream evidence based medicine is in some way accepted by mainstream science, and alternative medicine bears the “alternative” qualifier simply because it is not supported by mainstream science, then where does that leave a trader who seeks to refute any allegation that his claim is false?
Of course it is always open to the trader to show that his the alternative therapy actually works, but the weight of scientific evidence is likely to be against him.”
On the other hand, I’m advised by a Trading Standards Officer that “He doesn’t have to refute anything! The prosecution have to prove the claims are false”. This has been confirmed by another Trading Standards Officer who said
“It is not clear (though it seems to be) what difference is implied between “cure” and “treat”, or what evidence is required to demonstrate that such a cure is false “beyond reasonable doubt” in court. The regulations do not provide that the maker of claims must show that the claims are true, or set a standard indicating how such a proof may be shown.”
The main defence against prosecution seems to be the “Due diligence defence”, in paragraph 17.
Due diligence defence
17. —(1) In any proceedings against a person for an offence under regulation 9, 10, 11 or 12 it is a defence for that person to prove—
(a) that the commission of the offence was due to—
(i) a mistake;
(ii) reliance on information supplied to him by another person;
(iii) the act or default of another person;
(iv) an accident; or
(v) another cause beyond his control; and
(b) that he took all reasonable precautions and exercised all due diligence to avoid the commission of such an offence by himself or any person under his control.
If “taking all reasonable precautions” includes being aware of the lack of any good evidence that what you are selling is effective, then this defence should not be much use for most quacks.
Douglas McLachlan has clarified, below, this difficult question
False claims for health benefits of foods
A separate bit of legislation, European regulation on nutrition and health claims made on food, ref 1924/2006, in Article 6, seems clearer in specifying that the seller has to prove any claims they make.
Article 6
Scientific substantiation for claims
1. Nutrition and health claims shall be based on and substantiated by generally accepted scientific evidence.
2. A food business operator making a nutrition or health claim shall justify the use of the claim.
3. The competent authorities of the Member States may request a food business operator or a person placing a product on the market to produce all relevant elements and data establishing compliance with this Regulation.
That clearly places the onus on the seller to provide evidence for claims that are made, rather than the complainant having to ‘prove’ that the claims are false.
On the problem of “health foods” the two bits of legislation seem to overlap. Both have been discussed in “Trading regulations and health foods“, an editorial in the BMJ by M. E. J. Lean (Professor of Human Nutrition in Glasgow).
“It is already illegal under food labelling regulations (1996) to claim that food products can treat or prevent disease. However, huge numbers of such claims are still made, particularly for obesity ”
“The new regulations provide good legislation to protect vulnerable consumers from misleading “health food” claims. They now need to be enforced proactively to help direct doctors and consumers towards safe, cost effective, and evidence based management of diseases.”
In fact the European Food Standards Agency (EFSA) seems to be doing a rather good job at imposing the rules. This, predictably, provoked howls of anguish from the food industry There is a synopsis here.
“Of eight assessed claims, EFSA’s Panel on Dietetic Products, Nutrition and Allergies (NDA) rejected seven for failing to demonstrate causality between consumption of specific nutrients or foods and intended health benefits. EFSA has subsequently issued opinions on about 30 claims with seven drawing positive opinions.”
“. . . EFSA in disgust threw out 120 dossiers supposedly in support of nutrients seeking addition to the FSD’s positive list.
If EFSA was bewildered by the lack of data in the dossiers, it needn’t hav been as industry freely admitted it had in many cases submitted such hollow documents to temporarily keep nutrients on-market.”
Or, on another industry site, “EFSA’s harsh health claim regime”
“By setting an unworkably high standard for claims substantiation, EFSA is threatening R&D not to mention health claims that have long been officially approved in many jurisdictions.”
Here, of course,”unworkably high standard” just means real genuine evidence. How dare they ask for that!
Enforcement of the law
Article 19 of the Unfair Trading regulations says
19. —(1) It shall be the duty of every enforcement authority to enforce these Regulations.
(2) Where the enforcement authority is a local weights and measures authority the duty referred to in paragraph (1) shall apply to the enforcement of these Regulations within the authority’s area.
Nevertheless, enforcement is undoubtedly a weak point at the moment. The UK is obliged to enforce these laws, but at the moment it is not doing so effectively.
A letter in the BMJ from Rose & Garrow describes two complaints under the legislation in which it appears that a Trading Standards office failed to enforce the law. They comment
” . . . member states are obliged not only to enact it as national legislation but to enforce it. The evidence that the government has provided adequate resources for enforcement, in the form of staff and their proper training, is not convincing. The media, and especially the internet, are replete with false claims about health care, and sick people need protection. All EU citizens have the right to complain to the EU Commission if their government fails to provide that protection.”
This is not a good start. A lawyer has pointed out to me
“that it can sometimes be very difficult to get Trading Standards or the OFT to take an interest in something that they don’t fully understand. I think that if it doesn’t immediately leap out at them as being false (e.g “these pills cure all forms of cancer”) then it’s going to be extremely difficult. To be fair, neither Trading Standards nor the OFT were ever intended to be medical regulators and they have limited resources available to them. The new Regulations are a useful new weapon in the fight against quackery, but they are no substitute for proper regulation.”
Trading Standards originated in Weights and Measures. It was their job to check that your pint of beer was really a pint. Now they are being expected to judge medical controversies. Either they will need more people and more training, or responsibility for enforcement of the law should be transferred to some more appropriate agency (though one hesitates to suggest the MHRA after their recent pathetic performance in this area).
Who can be prosecuted?
Any “trader”, a person or a company. There is no need to have actually bought anything, and no need to have suffered actual harm. In fact there is no need for there to be a complainant at all. Trading standards officers can act on their own. But there must be a commercial element. It’s unlikely that simply preaching nonsense would be sufficient to get you prosecuted, so the Prince of Wales is, sadly, probably safe.
Universities who teach that “Amethysts emit high Yin energy” make an interesting case. They charge fees and in return they are “falsely claiming that a product is able to cure illnesses”.
In my view they are behaving illegally, but we shan’t know until a university is taken to court. Watch this space.
The fact remains that the UK is obliged to enforce the law and presumably it will do so eventually. When it does, alternative medicine will have to change very radically. If it were prevented from making false claims, there would be very little of it left apart from tea and sympathy
Follow-up
New Zealand must have similar laws.
Just as I was about to post this I found that in New Zealand a
“couple who sold homeopathic remedies claiming to cure bird flu, herpes and Sars (severe acute respiratory syndrome) have been convicted of breaching the Fair Trading Act.”
They were ordered to pay fines and court costs totalling $23,400.
A clarification form Douglas McLachlan
On the difficult question of who must prove what, Douglas McLachlan, who wrote Has The UK Quietly Outlawed “Alternative” Medicine?, has kindly sent the following clarification.
“I would agree that it is still for the prosecution to prove that the trader committed the offence beyond a reasonable doubt, and that burden of proof is always on the prosecution at the outset, but I think if a trader makes a claim regarding his product and best scientific evidence available indicates that that claim is false, then it will be on the trader to substantiate the claim in order to defend himself. How will the trader do so? Perhaps the trader might call witness after witness in court to provide anecdotal evidence of their experiences, or “experts” that support their claim – in which case it will be for the prosecution to explain the scientific method to the Judge and to convince the Judge that its Study evidence is to be preferred.
Unfortunately, once human personalities get involved things could get clouded – I could imagine a small time seller of snake oil having serious difficulty, but a well funded homeopathy company engaging smart lawyers to quote flawed studies and lead anecdotal evidence to muddy the waters just enough for a Judge to give the trader the benefit of the doubt. That seems to be what happens in the wider public debate, so it’s easy to envisage it happening a courtroom.”
The “average consumer”.
(3) A commercial practice is unfair if—
(a) it contravenes the requirements of professional diligence; and
(b) it materially distorts or is likely to materially distort the economic behaviour of the average consumer with regard to the product.
It seems,therefore, that what matters is whether the “average consumer” would infer from what is said that a claim was being made to cure a disease. The legal view cited by Mojo (comment #2, below) is that expressions such as “can be used to treat” or “can help with” would be considered by the average consumer as implying successful treatment or cure.
The drugstore detox delusion. A nice analysis “detox” at .Science-based Pharmacy
