cough
Which? Magazine (the UK equivalent of Consumer Reports in the USA) has done it again. They published an excellent article, Health products you don’t need. It’s a worthy successor to their recent debunking of “nutritional therapists”. Most of the products in question, apart from Bach Rescue Remedy Spray, were not outright quackery like homeopathy or "detox" products. Rather they were old-fashioned pharmaceutical products that were quite respectable in the 1950s but which have subsequently been found not to work.
These are the things that were looked at.
- Benylin Chesty Coughs (Non-Drowsy)
- Benylin Tickly Coughs (Non-Drowsy)
- Covonia Herbal Mucus
Cough Syrup - Boots Cold and Flu Relief
Tablets - Adios Slimming Tablets
- Bio-Oil
- Seven Seas Jointcare Be Active
- Bach Rescue Remedy
Spray
It’s an interesting and large category, and its one that I grew up with. My first job, in the 1950s was as an apprentice in Timothy Whites & Taylors Homeopathic Chemists in Grange Road, Birkenhead (you can’t have a much humbler start than that). Don’t worry about he homeopathic bit. We had one homeopathic prescription in two years, which was made up with great hilarity. These were the days before the endarkenment.
We did, however, sell a lot of "tonics" and "cough medicines". Two popular brands were Metatone Tonic and Minadex Tonic. I was quite surprised to discover that they are still on sale. Even in the 1950s I was a bit sceptical about what a "tonic" was supposed to achieve. The term soon became extinct as it was slowly realised that no examples existed.
Here is the bad news. It is scarcely an exaggeration to assert the following.
- Nothing is known that alters the time course of a cold.
- There is nothing that you can buy that will suppress a cough*.
- There is no such thing as a "demulcent" or an "expectorant"
- There is no such thing as a "tonic".
- It would be nice if these things existed, but they are figments of the imagination. Nonetheless they sell by the truckload and vast amounts of money are made by selling them.
[*morphine may have a modest effect, but you can’t buy it]
How can this happen? We have the Medicines and Healthcare products Regulatory Agency (MHRA) is the government agency which is responsible for ensuring that medicines and medical devices work, and are acceptably safe.
Demulcents
In the 1950s this was a more or less respectable term. If you google it now, almost all the references come from herbalists, It is, almost entirely, part of the world of quackery. Apart, that is, from the MHRA. And NHS Evidence. It was surprising to find, in a 2009 document from the MHRA
"Simple cough mixtures containing a demulcent, for example glycerin, and syrup can have a soothing effect by coating the throat and relieving the irritation which causes the cough"
No reference is given, and I’m not aware of the slightest reason to think that there is any such effect. Syrup in your respiratory tract is a bit of a disaster.
But the same document says
There are severe limitations to the efficacy studies given that many of the products were first introduced decades ago. There has been no co-ordinated development program to establish efficacy. What trials there are have not been carried out to current standards.
There isn’t much detail about these old ‘remedies’ on the MHRA site. I did find a Publiic Assessment Report for Benylin Mucus Cough Menthol Flavour Oral Solution. The main ingredient is Guaifenesin
The assessment says this.

And the approved label says this.

The Public Assessment Report also says
Efficacy
Guaifenesin . . . is a well-established medicinal product with well-known efficacy and safety profiles
This appears to be pure make-believe. There is no credible evidence for any such effect. The report may be dated 2012, but it is a carry-over from a previous age.
In 1976, it was pointed out that none of these things worked (Ziment, 1976), and the situation hasn’t changed. Ziment’s review concludes
"Perhaps this is the one disease that could truly benefit from that oft-touted panacea of therapeutics, the overworked nostrum of materia medica—namely, chicken soup"
A 2012 Cochrane review agrees: "Over-the-counter (OTC) medications for acute cough". This review concludes
"We found no good evidence for or against the effectiveness of over-the-counter (OTC) medications in acute cough, which confirms the findings of two previous reviews (Anonymous 1999; Smith 1993)."
What the MHRA tell me
I was puzzled by the apparently unjustified statements on the MHRA site so I asked them about the eight products that were investigated by Which? magazine (see above). I asked them also about Metatone and Minadex "tonics"
The questions, and the responses can be downloaded here. (I merely asked some reasonable questions, but the MHRA chose to treat them as a request under the Freedom of Information Act).
The first five items all have full marketing authorisation, as do Metatone and Minadex "tonics".
"Metatone (PL-02855/0017), Covonia Herbal Mucus Cough Syrup, Cold and Flu Relief Tablets and Adios Tablets originally held Product Licences of Right. These products were on the market before the Medicines Act 1968 came into force in 1971. These licences were reviewed in the 1980s to ensure that the products were safe, of suitable quality and have evidence of efficacy. Because of the length of time that the products had been on the market they were considered to have well established use and original clinical data to today’s standards was not necessarily available."
The MHRA tell me that they have no copies of the reviews conducted in the 1980s, apart from one. They sent a scanned copy of the August 1988 expert review of Covania syrup (the ingredients have changed since than).
The document is like an antique. It simply repeats the old myths. The names of the "expert reviewers" have been hidden. Given the quality of the review, perhaps that isn’t surprising, but the MHRA should not be so secretive.
There is no such thing as a "tonic", so I asked the MHRA about that too.
Q.5 Can you tell me what criteria the MHRA uses when allowing a product to be advertised as a "tonic"?
R.5 The MHRA assesses proposed product names on a case by case basis. On the basis of the well established use of Minadex Tonic it was decided that the use of the word tonic in the product name was acceptable. For the same reason, it was accepted that Metatone could be referred to as a tonic in the Product Information Leaflet and product labels
In other words, we let them get away with it because it’s old.
I had always understood that when the MHRA grants "Marketing authorisation", that meant there was some guarantee that the product worked. You’d infer that from the MHRA’s own definition.(my emphasis)
"Medicines which meet the standards of safety, quality and efficacy are granted a marketing authorisation (previously a product licence),"
Sadly, it seems that this isn’t true, at least for old-established products, those that were on the market before the Medicines Act (1968) came into force in 1971.
Conclusion
Although old products which were on the market before 1971 were supposed to be reviewed for efficacy and safety. This hasn’t been done efficiently. The make-believe has simply been perpetuated. I have no objection to people buying benylin etc, but they should not have full marketing authorisation and they should be labelled accurately so that it is clear that there is very little evidence that they’ll do you much good. The MHRA has let down the public, just as it did when it allowed misleading labels on homeopathic and herbal potions.
Postscript
After writing this, I discovered a very recent paper about guaifenesin (Seagrave et al, 2012), This paper shows some effects on mucus secretion in cultured human cells (not in humans) with prolonged exposure to concentrations of 30 and 100 µM. This is an order of magnitude greater than the peak blood concentration (7 µM. = 1.4 µg/ml) that is achieved (transiently) in man (Maynard & Bruce, 1970). This is not mentioned in the paper. I’m sure that has nothing to do with what we read at the end of the paper.
Competing interest
JS has received research funds from Reckitt Benckiser. HHA is a consultant to Reckitt Benckiser and is the co-author of a Mucinex sustained-release guaifenesin) patent. DBH has received research funds and consultancy payments from Reckitt Benckiser. DFR has received consultancy payments from Reckitt Benckiser. GS is an employee of Reckitt Benckiser and is also a co-author of a Mucinex (sustained-release guaifenfesin) patent.Acknowledgements
This study was funded by Reckitt Benckiser Healthcare International Ltd. Assistance with manuscript submission was provided by Elements Communications Ltd, supported by Reckitt Benckiser Healthcare International Ltd.
On 15 October 2010, Reckitt Benckiser was fined £10.2 m by the Office of Fair Trading after the company admitted anti-competitive behaviour.
Follow-up
Shortly after this post went up. I was attacked on twitter by @iHealthP. That’s a company, http://www.ihealthpartnership.com (the tweeter declined to reveal their identity). It started thus.
Your article asserts that “There is nothing that will suppress a cough.” This is bollocks, pure & simple.
The interchange was one of those less pleasant Twitter moments (I’ve Storifed some of it in case anyone is interested). The discussion did throw up a few useful references though. @LeCanardNoir pointed out a 2007 paper which concludes
"Clearly the widespread notion that codeine is an effective cough suppressant is not supported by the available evidence."
One of the papers cited by @iHealthP in support of his/her contention that pholcodine and codeine work was Recommendations for the management of cough in adults (from the British Thoracic Society Cough Guideline Group). This paper actually concludes
“There are no effective treatments controlling the cough response per se with an acceptable therapeutic ratio.”
That, of course, is exactly what I said.
There was, however, one reference produced by @iHealthP for which I’m grateful. It doesn’t concern over-the-counter cough treatments (which is what this post is about), but morphine. It does, though, produce some evidence that morphine does work to some extent as a cough suppressant. Amazingly this "well-known truth" was not demonstrated until 2006. The paper, Opiate Therapy in Chronic Cough, by Morice et al., 2006. shows a convincing effect of morphine (5 or 10 mg twice a day) on chronic cough. The main caveat lies in the reported side effects: constipation (40%) and drowsiness (25%). Obvious side-effects can make the trial non-blind. In any case, none of this is relevant to the present post (though I altered the blog to refer to it).
The press releases (STOP PRESS)
Uhuh, here we go again.
All over the media we see headlines like “Honey ‘beats cough medicine’ “.
Take for example, the Daily Telegraph, where Ben Farmer writes “Honey is better at treating children’s coughs than an ingredient used in many over-the-counter medicines, according to new research”.
That is NOT what the research found This is what the research paper itself says (DM refers to the standard ‘cough suppressant’ dextromethorphan, which is already known to be ineffective).
| “honey was significantly superior to no treatment for cough frequency’ DM was not better than no treatment for any outcome. Comparison of honey with DM revealed no significant differences.” |
See it? No detectable difference between honey and standard cough medicine.
Everyone in the media misinterpreted what the paper said, but at least one blogger is already on to it, with Today’s “duh” study is a honey”.
At first sight, the results seem contradictory, No difference between honey and DM, No difference between DM and ‘no treatment’. So how can honey be better than ‘no treatment’?
The study was by Ian M. Paul, MD, MSc; Jessica Beiler, MPH; Amyee McMonagle, RN; Michele L. Shaffer, PhD; Laura Duda, MD; Cheston M. Berlin Jr, MD, published in Archives of Pediatrics & Adolescent Medicine 2007, 161, 1140 – 1146.
What was done
The design of this trial was pretty good apart from one thing Three things were compared (a) buckwheat honey, (b) a standard ‘cough suppressant’, dextromethorphan in a honey-flavoured syrup that was designed to be similar to the honey (DM for short), and (c) no treatment whatsoever.
The median age of the children who completed the study was 5.2 years (range, 2.2 – 16.9 years). They all had coughs attributed to upper respiratory tract infection. Thirty-five patients received honey, 33 received DM, and 37 received no treatment.The good thing is that the treatments were allocated randomly to the children, and that the person doing the assessment didn’t know which treatment each child had received. The children didn’t know whether they were getting honey or DM either, but they DID know when they got ‘no treatment’. The trial was carried out over two days. On day one nobody got a treatment, but they filled in a survey that asked, for example, “How frequent was your child’s coughing last night”. The parent had to tick one of seven boxes, from ‘not at all’ (score zero) to ‘extremely’ (score 6). They were then given the treatment allocated to them in a brown paper bag, so the person who gave it didn’t know which it was. The patients then went home and on the next day the same survey was completed by the same parent, over the telephone.
What happened?
First look at the raw data. Here is Figure 2 from the original paper.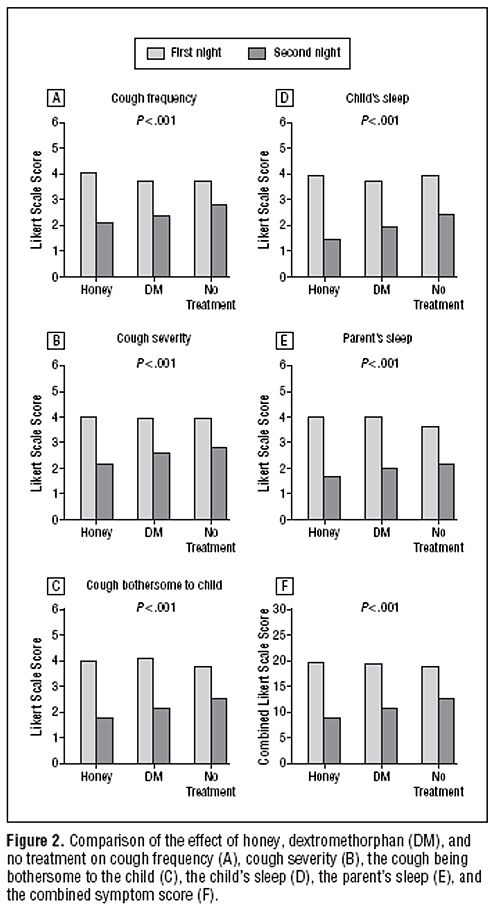 The charts show the results for 5 different measures of the severity of cough, and the last chart (F) shows the aggregate score for all the criteria.
The charts show the results for 5 different measures of the severity of cough, and the last chart (F) shows the aggregate score for all the criteria.
The first thing to notice is that there are no error bars on these charts. In my area, at least, no journal would accept a chart like this with no indication of scatter. There is a snag, though. Each patient acts as his/her own control, and that would not be reflected properly if errors bars were calculated for the numbers plotted in Fig. 2. It would therefore have been better to have a chart in which the difference in score between day 1 and day 2 was calculated from each patient, and the size of these differences plotted, with a standard deviation of the mean to indicate the amount of scatter in the observations. I have asked Dr Paul to send me a version that indicates the scatter of the numbers in this way (but I don’t think it will come).
The second thing to notice is that there is there is quite a big difference between the score on the first day (pale columns) and on the second day (dark columns), even in the no treatment group .
Thirdly, the pale columns are all much the same. On the first day the average score was about 4 (“a lot”) though on the second day, even with no treatment, the score fell quite a lot, to something between 2 (“a little”) and 3 (“somewhat”). This is a bit baffling because no treatment was given on either day. Presumably it results from the different settings in which the survey was given, or because the kids were getting better anyway.
Fourthly, insofar as the pale columns (baseline values) are all much the same, the thing you need to concentrate on is the difference, on each chart, between the height of the dark bars, for honey, DM and no treatment. These differences are pretty small, but on all the charts, the honey score is slightly smaller than the DM score, and the DM score is slightly smaller than the ‘no treatment’ score. What are we to make of that?
Here beginneth the statistical lesson.
Because the differences are small, and the scatter is quite big, we have to ask whether the differences are just random fluctuations rather than a result of any real difference between the treatments. That means we need statistics. Here is how the statistical argument works. Put roughly, we ask “how probable is it that the observations could arise by chance”. More precisely, the question is this. If there were no difference between the treatments, what is the probability that we would observe by chance a difference as big as, or bigger than, that seen in the experiment? (You need the subjunctive mood to explain statistics -pity it’s vanishing.)
Above each chart in the Figure we see P < 0.001. This means that there is less than a one in 1000 chance of the results arising by chance. More precisely, if all three treatments (honey, DM and no treatment) were actually identical, it is very unlikely that we’d see these results. The reasonable conclusion is, therefore, that all three treatments are not identical. The problem with this argument is that it tells you nothing about where the differences lie, so it is of no help whatsoever to a patient who is trying to decide what to do about a cough. The other problem is that it includes the ‘no treatment’ group, which was not blind. Both the children and parents were well aware that no treatment was given.
The most helpful comparison is really the properly-blinded comparison between honey and DM. And when this was looked at the result was no significant differences. In other words the small differences between the heights of the dark columns for honey and DM could perfectly well have arisen by chance if honey and DM were identical in their properties.
There isn’t any reason at all to think that honey is better than the standard (but ineffective) cough medicine.
The direct comparison between DM and ‘no treatment’ also shows no significant difference. Yet there are signs of a real difference between ‘no treatment’ and honey, though only for the cough frequency, not the other four measures. The aggregate measure (F in the figure) gave P = 0.04 for the comparison, so the authors are running a risk of 1 in 25 of being wrong in claiming a real effect. Although some people seem to regard a value of P = 0.05 as indicating a real effect, the fact that you’ll make a fool of yourself 1 time in 20 by claiming a real effect when none exists has never seemed to me to be good enough odds to stake one’s reputation on.
The ‘no treatment’ group certainly has some interest, but the fact that it was not blind means that the fact that honey was marginally better than ‘no treatment’ could perfectly well mean that taking honey has a better placebo effect that doing nothing at all. It provides no evidence at all that honey has any genuine therapeutic effect. If it had, one would then have to find out if the therapeutic effect was specific to buckwheat honey, or whether any old honey would do. It could be argued that even if the effect were real rather than placebo, the size of the effect is too small to make all that effort worthwhile.
A couple more things
It is already well known, from several good studies, that DM is useless, no better than placebo. This inconvenient fact has not yet reached many places that it should have (not even mentioned on wikipedia for example), but the American Academy of Pediatrics says
“Numerous prescription and nonprescription medications are currently available for suppression of cough, a common symptom in children. Because adverse effects and overdosage associated with the administration of cough and cold preparations in children have been reported, education of patients and parents about the lack of proven antitussive effects and the potential risks of these products is needed.”
The discussion in the paper by Paul et al, seems surprisingly upbeat about honey, in the light of their own findings. I’m surprised that they use the term ‘demulcent’ which I had thought to have died out, like the word ‘tonic’, on the grounds that it had no defined meaning
It is because meaningless terms and useless medicines die out eventually that medicine makes progress. The problem with alternative medicine is that nothing dies out: on the contrary they keep adding myths.
And finally
Always look at the end of the paper. On this one we see that the study was paid for by the National Honey Board. Dr Paul assures me that the funding source had no say in the design or analysis, which is as it should be.
Financial Disclosure: Dr Paul has been a consultant to the Consumer Healthcare Products Association and McNeil Consumer Healthcare.
Funding/Support: This work was supported by an unrestricted research grant from the National Honey Board, an industry-funded agency of the US Department of Agriculture.
So what is the practical outcome?
My conclusion from all this is simple. If you have got a cough, tough luck. There isn’t really anything available, conventional or alternative, that does much good. You’ll just have to wait for it to get better. But if you want to take something that tastes nice, why not honey? It almost certainly won’t do any good but it tastes good and it’s safer than the standard cough medicine.
The sponsor’s interpretation
It seems that the sponsor of the work is happy with the misinterpretation.
Charlotte Jordan a project manager of research at the National Honey Board, believes the finding confirms what your grandmother told you.
“This is a really exciting finding,” she said. “For a long time it’s been folklore medicine to use honey when you have a cough or a cold, but it’s exciting to have a scientific study to back that up.”
Just one problem, That is NOT what the paper says.
How did all this mis-reporting happen?
One reason is misleading press releases. Universities and Academic journals now engage in shameless PR, spin and hype. They prostitute good science.
Download press releases from Penn State, JAMA and Press Association [pdf file]
Here is the highly misleading bit of hype that came from the Press Office of the Pennsylvania State University. The headline is “Honey a better option for childhood cough than OTCs” (OTC means over-the-counter medicines that contain DM). That contradicts directly the paper which says “Comparison of honey with DM revealed no significant differences”.
Likewise the statement in the Penn State release “Honey did a better job reducing the severity, frequency and bothersome nature of nighttime cough from upper respiratory infection than DM or no treatment” is equally incompatible with “Comparison of honey with DM revealed no significant differences”. Its only possible justification is from the 3 way comparison by analysis of variance and that does not tell us what we need to know.
To make matters worse, the media office is not to blame this time. Ms Manlove told me tonight that the press release had been approved by Dr Paul himself.
| Contact: Megan W. Manlove Penn State Honey a better option for childhood cough than OTCs A new study by a Penn State College of Medicine research team found that honey may offer parents an effective and safe alternative than over the counter children’s cough medicines. The study found that a small dose of buckwheat honey given before bedtime provided better relief of nighttime cough and sleep difficulty in children than no treatment or dextromethorphan (DM), a cough suppressant found in many over-the-counter cold medications. Honey did a better job reducing the severity, frequency and bothersome nature of nighttime cough from upper respiratory infection than DM or no treatment. Honey also showed a positive effect on the sleep quality of both the coughing child and the child’s parents. DM was not significantly better at alleviating symptoms than no treatment. . . . |
All that Candice Yakel, of the Office for Research Protections at Penn State had ro say in the matter was
“Our investigators stand by the conclusions of the study as reported in the Archives of Pediatric and Adolescent Medicine and as characterized in our press release of December 3, 2007.”
And here is the equally misleading bit of hype issued by the Journal of the American Medical Association (Ms Manlove tells me that this was also approved bt Dr Paul).
| JAMA and Archives Journals Study suggests honey may help relieve children’s cough, improve sleep during colds
|
The Press Association release was equally bad, and probably the one used by many of the reporters as a basis for stories in the media. The opening statement is totally wrong.
| 1 HEALTH Honey Embargoed to 2100 Monday December 3 HONEY BEST FOR KIDDIES’ COUGHS SAY RESEARCHERS By John von Radowitz, PA Science Correspondent Natural honey is a better remedy for children’s coughs than expensive over-the-counter medicines, researchers said today. A dose of buckwheat honey before bedtime easily outperformed a cough suppressant widely used in commercial treatments, a US study found. . . . |
Follow-up
There is a review of over-the-counter cough medicines in the BMJ (2002) [free full text]. It concludes “Recommendation of over the counter cough medicines to patients is not justified by current evidence”.


